Surface melting in 3D
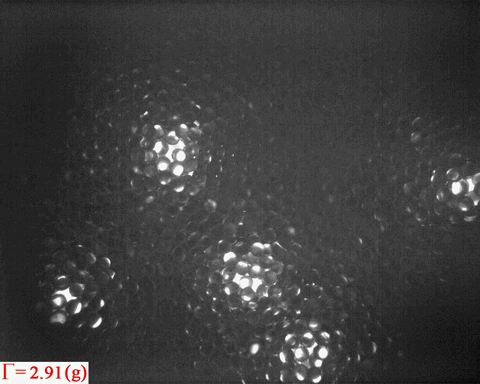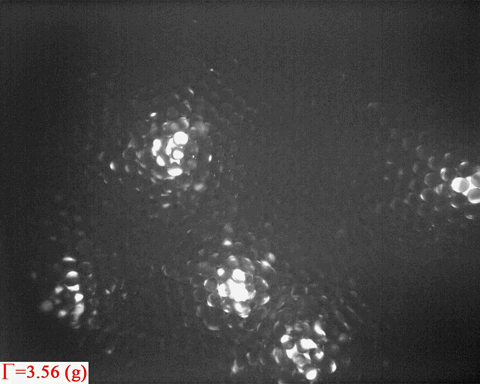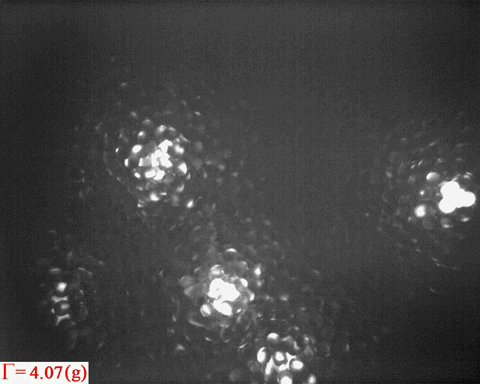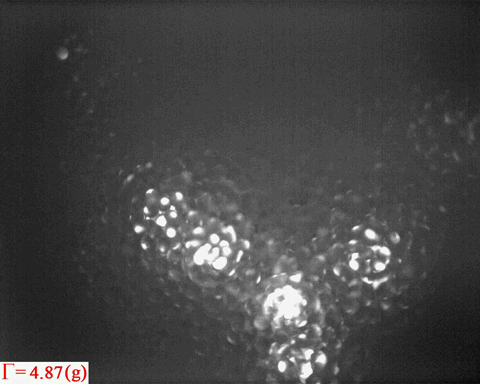
Bright patterns are from the light emitted from Ruby tracers, which are
embedded in the wet granular sample as peak acceleration increases
step by step
|
Phase separation

As the energy injection is sufficient to break capillary bridges
between adjacent particles, a wet granular 'gas bubble' emerges.
|
Surface melting has been a topic of interest since
Michael Faraday's observations on regelation, welding of two blocks of
ice after contact below 0 degree. After more than a century's
investigations, it becomes clear that melting is a continuous process
that tends to start from the free surface (see e.g. a review of premelted ice).
In a non-equilibrium system such as wet granular matter, it is obvious
from the above video that melting also tends to starts from the
surface. Again, the question is:
- How does the analogue of surface melting in a random packing of spheres compare to that in thermal systems?
|
Merging of wet granular 'gas bubbles' suggests the
existence of interfacial tension. The questions are:
- How to define the surface tension in such a nonequilibrium system?
- From the fluctuations of gas bubbles, can we learn something about viscousity of a wet granular liquid?
|
![]() German
German ![]() German
German